Abstract
Background: Ponatinib has been established as an optimal salvage treatment option in chronic myeloid leukemia (CML) patients who fail to second generation tyrosine kinase inhibitors (2GTKIs) or harbor T315I mutations. However, serious adverse events, mainly cardiovascular (CVS) events, have been related to ponatinib use at the standard dose (45 mg/day), and therefore dose reductions have been suggested for those patients achieving optimal responses. Clinical trials are currently evaluating different dose schemes in order to answer whether dose modifications could diminish side effects while maintaining responses.
Aims: To evaluate efficacy and safety of ponatinib after dose modifications recommendations in the real life-setting.
Methods: We have performed a multicenter observational retrospective study collecting information from CML patients treated with ponatinib between 2014 and 2016 in 32 Spanish centers. The indication of ponatinib, as well as its dose schedule to mitigate the risk of cardiovacular toxicity, was made according to the criterion of the attending physician on the basis of current clinical recommendations. Data collection followed regulations for observational studies. Patients were analyzed by intention-to-treat. Molecular biology tests were performed according to ELN guidelines and BCR-ABL/ABL are expressed as % IS in all centers. Standard clinical and comorbidities information (including cardiovascular risk factors) were collected before and during ponatinib treatment. For progression free survival (PFS) calculation, progression was defined as transformation to advances phases and event for event free survival (EFS) as treatment discontinuation due to any reason, progression or death.
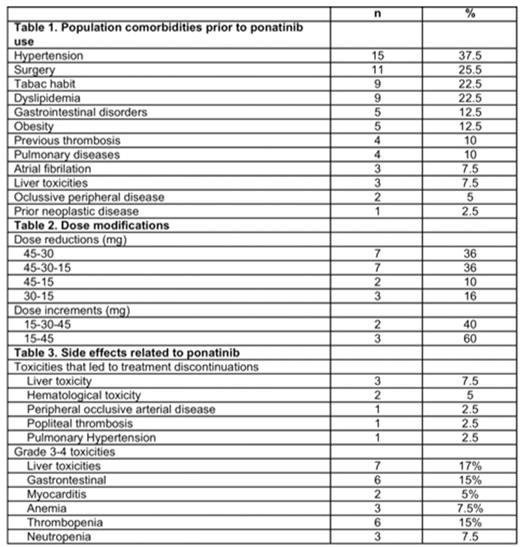
Results: A total of 40 CML patients were included in the study. Patients had received a median of 2.7 TKIs before prior to ponatinib treatment, with a median time of 39 months from diagnosis to ponatinib start. Median age at diagnosis was 58 years and Sokal prognostic index was 26%, 31% and 28% for low, intermediate and high respectively. 87% patients were diagnosed in chronic phase, 8% in accelerated and 5% in blastic phase. Most frequent comorbidities before ponatinib was started are shown in table 1. In total, 67% had cardiovascular risk factors at the time of starting with ponatinib and 4 patients (10%) hade suffered previous thrombosis. Most frequent BCR-ABL mutations were T315I and G250E found in 44% and 11% of the patients. Seventeen percent of the patients harbored 2 or more mutations. All patients started with ponatinib at 45mg daily, but 20 of them (50%) had dose modifications due to occurring side effects or in order to prevent them (scheme doses is shown in table 2). Overall, the cumulative incidence of complete hematological response, complete cytogenetic responses, major molecular responses and MR4.5 were 100%, 57%, 47% and 28%, respectively. Overall, 47% of the patients improve previous responses, 39% maintained the same degree of response and 14% lost response. After a median follow-up of 18 months, 20 patients (50%) discontinued treatment due to adverse events (40%), bone marrow transplantation (25%), progression to advances phases (15%), lack of efficacy 5%), death (5%) or unknown reasons (5%). Most frequent toxicities leading to treatment discontinuations were severe cardiovascular events (peripheral occlussive disease, pulmonary hypertension and deep vein thrombosis), hepatotoxicity, and myelotoxicity. The list of adverse events during ponatinib treatment is shown in table 3. SLE, PFS and overall survival was 45%, 72% and 85% respectively with a median follow up of 18 months.
Conclusions: In the real life setting, the strategy of limiting ponatinib exposure in responding patients appears to be safe while maintaining the clinical efficacy.
García Gutiérrez: Novartis: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Incyte: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding. Jimenez-Velasco: BMS: Consultancy; Pfizer: Consultancy; Incyte: Consultancy; Novartis: Consultancy. Casado Montero: Pfizer: Consultancy, Honoraria, Research Funding; Incyte: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Research Funding. Sanchez-Guijo: Incyte: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria. Gomez-Casares: BMS: Speakers Bureau; Pfizer: Speakers Bureau; Novartis: Speakers Bureau; Incyte: Speakers Bureau. Vallansot: Novartis: Honoraria, Research Funding; BMS: Honoraria; Ariad: Honoraria. Steegmann: Incyte: Consultancy, Honoraria, Speakers Bureau; BMS: Consultancy, Honoraria, Research Funding, Speakers Bureau; Pfizer: Consultancy, Honoraria, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal